what is the main use of hydrocarbons in crude oil Hydrocarbons crude
Crude oil is a vital natural resource that plays a significant role in powering our modern world. It is a complex mixture of hydrocarbons, which are organic compounds made up of hydrogen and carbon atoms. Hydrocarbons can be divided into different classes based on their molecular structures and properties, each with its unique characteristics and uses.
Crude Oil and Hydrocarbons: An Overview
Crude oil, also known as petroleum, is a fossil fuel that is formed from the remains of ancient marine organisms over millions of years. It is found deep beneath the Earth’s surface and extracted through drilling techniques. Crude oil is a dark, viscous liquid that varies in color and composition depending on its source.
Hydrocarbons are the main components of crude oil, accounting for around 90-95% of its composition. They can be classified into different classes based on their carbon chain length and structure. These classes include alkanes, cycloalkanes, alkenes, alkynes, and aromatic hydrocarbons.
Understanding Hydrocarbon Classes
 Alkanes: Also known as saturated hydrocarbons, alkanes possess single bonds between carbon atoms. They have the general formula CnH2n+2. Alkanes are known for their stability and are commonly used as fuels, including gasoline and diesel.
Alkanes: Also known as saturated hydrocarbons, alkanes possess single bonds between carbon atoms. They have the general formula CnH2n+2. Alkanes are known for their stability and are commonly used as fuels, including gasoline and diesel.
Cycloalkanes: Cycloalkanes are hydrocarbons with carbon atoms arranged in a ring structure. They are often used as solvents and are important in the production of various chemicals and polymers.
Alkenes: Alkenes contain at least one carbon-carbon double bond, giving them a distinct reactivity. They are utilized in the production of plastics, synthetic fibers, and as starting materials for numerous chemical reactions.
Alkynes: Alkynes possess at least one carbon-carbon triple bond. They have unique properties and are commonly used in chemical synthesis and the manufacturing of special materials, such as polymers and pharmaceuticals.
Aromatic Hydrocarbons: Aromatic hydrocarbons feature a unique ring structure called a benzene ring. They are widely used as solvents, fuels, and in the production of plastics, dyes, and pharmaceuticals.
The diversity of hydrocarbon classes in crude oil allows for the production of a wide range of products that are crucial in our daily lives. From fuels and lubricants to plastics, chemicals, and pharmaceuticals, hydrocarbons serve as the building blocks of numerous industries.
As our understanding of crude oil and its hydrocarbon composition continues to advance, so does our ability to develop innovative technologies for its extraction, refining, and utilization. The exploration of alternative and renewable energy sources is also gaining traction globally to mitigate environmental impacts and reduce dependence on finite fossil fuels like crude oil.
In conclusion, crude oil and its hydrocarbons are essential resources that have shaped our modern world. Understanding its composition and the different classes of hydrocarbons within it is crucial for optimizing its use, developing new technologies, and ensuring sustainable energy practices.
If you are looking for Adams Blog: 5.9 I can describe the trend in boiling points and you’ve came to the right page. We have 5 Pictures about Adams Blog: 5.9 I can describe the trend in boiling points and like GCSE Chemistry - Crude Oil and Hydrocarbons, what is Crude Oil and how, savvy-chemist: GCSE OCR Gateway Organic Chemistry C6.2l and n Crude Oil and also Adams Blog: 5.9 I can describe the trend in boiling points and. Here it is:
Adams Blog: 5.9 I Can Describe The Trend In Boiling Points And
 adamblogscience.blogspot.comfractions boiling trend oil crude adams point
adamblogscience.blogspot.comfractions boiling trend oil crude adams point
GCSE Chemistry - Crude Oil And Hydrocarbons, What Is Crude Oil And How
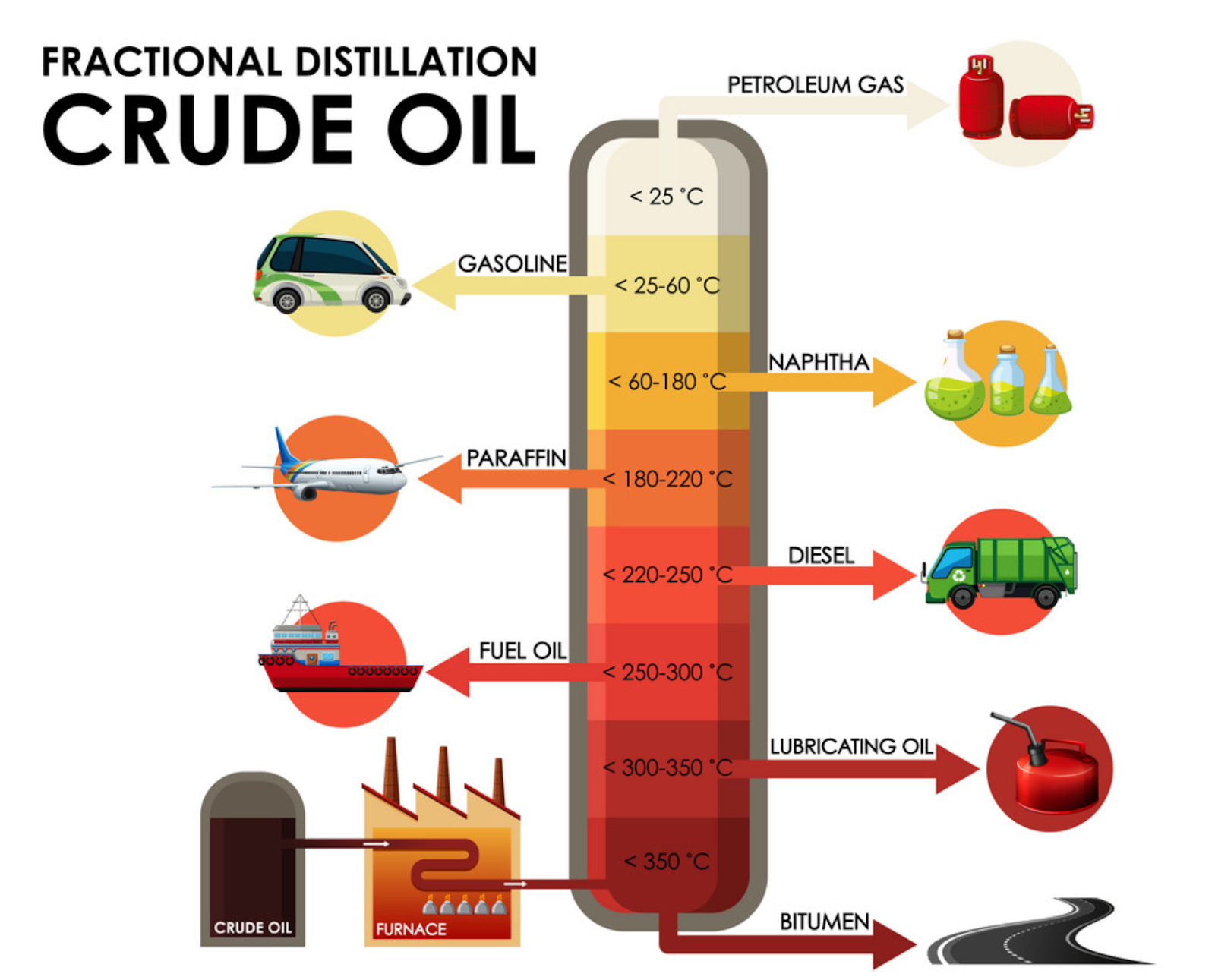 sherpa-online.comSavvy-chemist: GCSE OCR Gateway Organic Chemistry C6.2l And N Crude Oil
sherpa-online.comSavvy-chemist: GCSE OCR Gateway Organic Chemistry C6.2l And N Crude Oil
 derekcarrsavvy-chemist.blogspot.comcrude oil hydrocarbons where chemistry plastics barrel petroleum breakdown components percentages source extracted refined chemist earth life into gcse savvy
derekcarrsavvy-chemist.blogspot.comcrude oil hydrocarbons where chemistry plastics barrel petroleum breakdown components percentages source extracted refined chemist earth life into gcse savvy
Hydrocarbons Classes From A Crude Oil | Download Scientific Diagram
 www.researchgate.nethydrocarbons crude
www.researchgate.nethydrocarbons crude
Limestone,oil, Fractionational Distillation - Presentation Chemistry
.PNG)
Adams blog: 5.9 i can describe the trend in boiling points and. Hydrocarbons crude. Limestone,oil, fractionational distillation